Cuelgo este documento aquí, en el este foro, porque entiendo que a muchos de nosotros, entre otras muchas goteras que desencadenan el SFC, se nos ha dicho que tenemos ( en mi caso confirmado con analítica) o que podemos tener el síndrome de permeabilidad intestinal.
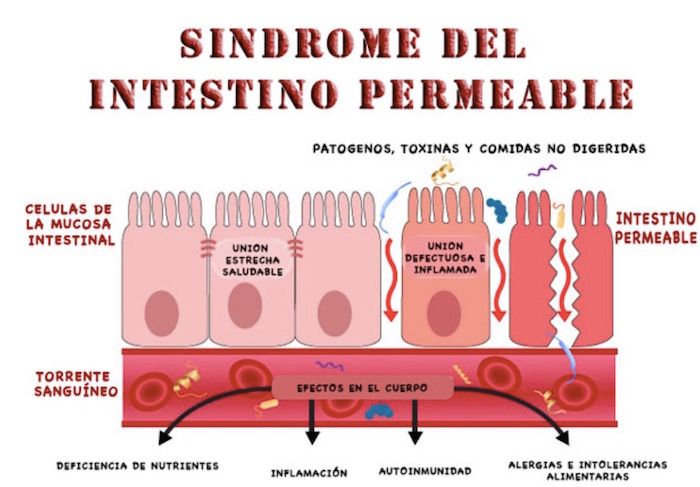
Dicho documento, de fecha septiembre de 2014, elaborado por el Dpto. de Patología de la Universidad de Chicago, vendría a desmentir, o poner en duda, la existencia de dicho sindrome, y que paso a publicar:
Intestinal permeability defects: Is it time to treat?
Matthew A. Odenwald and Jerrold R. Turner
Department of Pathology, The University of Chicago
Abstract
An essential role of the intestinal epithelium is to separate luminal contents from the interstitium, a function primarily determined by the integrity of the epithelium and the tight junction that seals the paracellular space. Intestinal tight junctions are selectively-permeable, and intestinal permeability can be increased physiologically in response to luminal nutrients or pathologically by mucosal immune cells and cytokines, the enteric nervous system, and pathogens. Compromised intestinal barrier function is associated with an array of clinical conditions, both intestinal and systemic. While most available data are correlative, some studies support a model where cycles of increased intestinal permeability, intestinal immune activation, and subsequent immune-mediated barrier loss contribute to disease progression. This model is applicable to intestinal and systemic diseases. However, it has not been proven and both mechanistic and therapeutic studies are ongoing. Nevertheless, the correlation between increased intestinal permeability and disease has caught the attention of the public, leading to a rise in popularity of the diagnosis of “leaky gut syndrome,” which encompasses a range of systemic disorders. Proponents claim that barrier restoration will cure underlying disease, but this has not been demonstrated in clinical trials. Moreover, human and mouse studies show that intestinal barrier loss alone is insufficient to initiate disease. It is therefore uncertain if increased permeability in these patients is a cause or effect of the underlying disorder. Although drug targets that may mediate barrier restoration have been proposed, none have been proven effective. As such, current treatments for barrier dysfunction should target the underlying disease.
A critical function of the intestinal mucosa, particularly the epithelium, is to form a barrier that prevents potentially noxious contents of the intestinal lumen, including the microbiota, from accessing internal sites and the systemic circulation.1 Barrier defects have been reliably associated with a variety of human diseases, including those primarily affecting the gut, e.g. inflammatory bowel disease (IBD), celiac disease, and irritable bowel syndrome, as well as systemic diseases or diseases involving other organ systems, e.g. type I diabetes, graft versus host disease (GVHD), HIV, multiple sclerosis, rheumatoid arthritis, and autism. The topic is further confused by use of the term “leaky gut syndrome” within the lay and alternative medicine communities, and even by some physicians, and claims that this is responsible for a dizzying array of disorders, including chronic fatigue syndrome, fibromyalgia, allergies, depression, and skin disorders (Table I). In part, the speculation and uncertainty regarding both bona fide disease associations and those ascribed to leaky gut syndrome reflect the absence of conclusive human data. To date, all clinical studies have focused on correlation, precluding distinction between cause and effect. Further, no therapies to directly target and restore the intestinal barrier are currently available as FDA-approved or even investigational drugs. Here, we review the determinants of intestinal barrier function, real and claimed associations with disease, experimental data that may shed light on the pathogenic contributions of intestinal permeability defects, and the potential of future advances to provide therapeutic tools for barrier restoration.
Technological Primer
Definitions
Intestinal permeability is the property that allows solute and fluid exchange between the lumen and tissues. Conversely, intestinal barrier function refers to ability of the mucosa and extracellular barrier components, e.g. mucus, to prevent this exchange. Neither permeability nor barrier function is absolute, and the relative magnitudes of these opposing characteristics vary inversely. However, the term intestinal barrier has become a catch-phrase that is being increasingly applied to related, but distinct, properties, e.g. immunoregulatory and antibacterial barriers. While these may be barriers, they should not be characterized as permeability or barrier function. To avoid confusion caused by the pervasive indiscriminate use of these terms it is critical to first define them. In this article we will use barrier function as the converse of intestinal permeability, and both will refer exclusively to changes in flux of solutes and fluids across the epithelium.
The small intestinal and colonic mucus layers,2 which are composed of goblet cell secretions, form the first barrier. The apical mucus also contributes to the development of the zone of limited luminal flow, i.e. the unstirred layer,3 that directly overlies the epithelium. While the mucus and unstirred layers prevent some organisms and large molecules, e.g. food particles, from directly accessing the epithelium, they do little to prevent flux of small molecules, ions, and water. Nevertheless, studies using experimental animals have shown that disruption of mucus production can lead to intestinal damage and inflammatory disease.4
Beneath the mucus and unstirred layers lies a simple columnar epithelium. The membranes of these epithelial cells provide an effective barrier to most hydrophilic solutes, but would not be an adequate barrier if the space between individual cells were not sealed by a series of intercellular junctions. Of these, the tight junction is the primary determinant of paracellular flux in an intact epithelium.5 As discussed below, intestinal tight junctions do not form an absolute seal. It should also be apparent that disruptions of intestinal barrier function, i.e. increased permeability, due to direct epithelial damage do not reflect tight junction barrier function.
Permeability pathways
The tight junction is a highly dynamic, protein complex that forms within specialized plasma membrane lipid domains.6, 7 The specific proteins involved have been reviewed elsewhere6, 8–11 and, except where linked to disease, individual tight junction proteins will not be discussed here. It has been recognized for many years that tight junctions restrict molecular flux on the basis of both size and charge, i.e. tight junctions display size- and charge-selectivity. 12–15 Recent in vitro and in vivo studies using cultured human intestinal epithelia and mice, respectively, have shown that two disease-related cytokines tumor necrosis factor (TNF) and interleukin-13 (IL-13) can differentially regulate tight junction size- and charge-selectivity (Fig. 1a).16, 17 Together with high resolution in vitro analyses of tight junction size selectivity,18–21 this has led to a model in which there are at least two distinct routes of paracellular flux across the tight junction.1, 6, 10 These have been termed pore and leak pathways and refer to high capacity, size-and charge-selective and a low capacity, nonselective routes, respectively.1, 10 IL-13 specifically increases flux across the pore pathway by upregulating expression of the tight junction protein claudin-2, which forms a paracellular cation and water channel.17, 22–24 In contrast, TNF increases leak pathway flux by a rapid mechanism that involves reorganization of the tight junction and perijunctional actomyosin ring by a process that requires myosin light chain kinase (MLCK).16, 25 Notably, claudin-2 expression and MLCK activity are both increased in active IBD,22, 26 suggesting that both pathways are involved in disease-associated barrier loss.
Measures of intestinal permeability
Many tools exist to measure intestinal permeability ex vivo and in vitro.6, 27 While simple in vivo methods are also available and readily-applicable to human subjects and experimental animals, they offer less resolution and interpretation can be complex. These approaches most frequently assess fractional urinary excretion of orally-ingested probes (Fig. 1b).28 The probes selected must not be able to cross the epithelium by a transcellular route, and any probe that enters the blood stream is assumed to have crossed the tight junction or a site of epithelial damage. Probes must also be inert within the blood stream and freely-filtered at the glomerulus, which allows their collection in the urine. However, many factors, including intestinal transit time, intestinal surface area, probe degradation within the lumen or bloodstream, and renal function can affect fractional urinary excretion.29, 30 It is therefore not surprising that a variety of probes have been used and that each has a unique constellation of limitations.
For measurements of small intestinal permeability, the lactulose:mannitol ratio (sometimes referred to as LAMA) has been popular. The use of two probes in this case partially corrects for absorptive differences resulting from changes in motility. However, lactulose and mannitol are degraded by colonic bacteria28 and are not useful as measures of colonic permeability; probes including sucralose, polyethlylene glycols, and 51Cr-EDTA have been used instead. None of these probes are able to assess charge-selectivity.
Lactulose is large and can only cross via the leak pathway or at sites of epithelial damage. It can therefore be considered a marker of barrier integrity. Mannitol, which is one third as large, crosses the pore pathway and can be thought of as measure of surface area. Thus, the lactulose:mannitol ratio can be interpreted as a measure of the sum of leak pathway permeability and epithelial damage normalized to surface area. For example, mannitol recovery is reduced in celiac disease, reflecting loss of villous surface area, while lactulose recovery is increased due to epithelial damage. This results in an increased lactulose:mannitol ratio that is only partly due to increases intestinal permeability. Thus, it is critical to assess both lactulose and mannitol recoveries separately, as well as the lactulose:mannitol ratio, when interpreting test results.
Remarkably, several internet vendors promoting leaky gut syndrome treatments also provide mail-order lactulose-mannitol assays. Thus, patients may arrive with their own lactulose:mannitol data. However, as discussed below, it is not yet clear how these data can or should be used to guide diagnosis or treatment.
Findings
Physiological regulation of intestinal barrier function
The most studied instance of physiological intestinal barrier regulation is that triggered by Na+-glucose cotransport. This leads to activation of epithelial myosin light chain kinase (MLCK), which drives size-selective, i.e. pore pathway, increases in paracellular permeability.15, 31–33 This enhances pararcellular water flux as a result of the osmotic gradient created by transcellular Na+ and glucose transport. This combination of paracellular water flow and increased paracellular permeability also allows paracellular absorption of nutrient-sized molecules, such as glucose, that can reach very high concentrations within the unstirred layer as a result of brush border digestive enzyme activity. Thus, nutrients are carried passively as solutes within water, i.e. the solvent.34 This process, termed solvent drag, likely explains the inability of excess luminal glucose to saturate intestinal absorption. This mechanism also contributes to the efficacy of Na+- and carbohydrate-based oral rehydration solutions.35 In contrast, Na+-glucose cotransport-dependent barrier regulation has not been described in the kidney, where tubular reabsorption is saturated at glucose concentrations exceeding ~300 mg/dL and leads to spilling in the urine, e.g. in diabetes..
Disease correlates in human subjects
Intestinal permeability has been most extensively studied in the context of inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS). As noted above, the lactulose:mannitol ratio is most relevant for analysis of small intestinal permeability, thus most IBD studies have focused on Crohn’s disease (CD) patients with small intestinal disease. This limitation of the lactulose:mannitol ratio, as well as the impact of intestinal motility and transit time on probe absorption, complicate studies of IBS. Most importantly, while permeability by these measures is increased in CD, IBS, and other diseases, it is well recognized that disease processes, such as proinflammatory cytokine release, can impact intestinal permeability. This makes it impossible to separate cause from effect. The strongest case for a pathogenic role of barrier loss has been made in CD, where some healthy first- degree relatives of CD patients exhibit increased intestinal permeability compared to unrelated control subjects.36, 37 Subsequent studies showed that these healthy relatives with increased permeability tended to carry NOD2 mutations,38 suggesting that the increased permeability observed reflects subclinical immune activation. Unfortunately, we do not know if these healthy relatives with increased permeability are at greater risk of developing disease than the healthy relatives with normal intestinal permeability.
The intestinal barrier may also be critical to maintenance of CD remission, as increased intestinal permeability can be a strong risk factor for CD reactivation.39, 40 The factors that contribute to increased permeability during remission have not been defined, but could include psychological stress, which has been associated with CD relapse41 and has also been shown to induce intestinal barrier loss in rodents.42 However, the data can also be interpreted as evidence of a mild inflammatory state and smoldering disease that is poised to become clinically active (Fig. 1a). Despite these uncertainties, it should be clear that barrier loss alone is insufficient to cause CD or any other disease. Indeed, data from some mouse studies indicate that mild permeability increases may activate immunoregulatory pathways that can limit colitis upon subsequent challenge.1, 43
Importance
Implications of animal model data for human disease
Intestinal barrier loss, i.e. permeability increases beyond the normal range, precedes disease onset in mouse models of type I diabetes, GVHD, necrotizing enterocolitis, IBS, and IBD. Unfortunately, like human data, most studies using these models have failed to distinguish between cause and effect. There are, however, several exceptions. For example, a zonulin antagonist (larazotide) has been shown to reduce diabetes and IBD in the non-obese diabetic and IL-10 knockout mouse models of these diseases.44, 45 Regrettably, clinical results have shown that larazotide does not prevent gluten-induced permeability increases in celiac disease patients.46
A range of experimental models have been used to study disease-associated barrier loss. In these models it is important to distinguish between tight junction-dependent and tight junction- independent barrier loss. For example, the commonly used dextran sulfate sodium (DSS) model of colitis causes direct colonic epithelial cell injury and death. While there is documented tight junction disruption in this disease, the bulk of observed barrier loss is due to epithelial loss. This barrier loss is far greater in magnitude than that resulting from tight junction dysregulation. Thus, while DSS is an excellent model of acute intestinal damage, it does not reflect IBD, IBS, or celiac disease in mechanistic terms, and therapies effective in DSS colitis have failed in human IBD trials. The most relevant information may come from mouse models that employ mechanisms similar to those activated in human disease. In IBD, these include the IL-10 knockout mouse and models that rely on adoptive transfer of effector T lymphocytes into immunodeficient mice that lack essential immunoregulatory processes. Regardless, it is critical to assess the model used when determining relevance to human disease pathogenesis.
One disease model that may provide insight into the role of intestinal permeability in disease initiation and propagation is the constitutively active-myosin light chain kinase (CA-MLCK) mouse. In this mouse, CA-MLCK within the epithelium induces intestinal epithelial tight junction barrier loss without associated epithelial damage.47 This increased intestinal permeability to a degree similar to that seen in healthy first-degree relatives of CD patients.36, 48 It is therefore not surprising that CA-MLCK transgenic mice failed to develop spontaneous disease. However, they did display subclinical immune activation. Further, CA- MLCK transgenic mice developed a more severe colitis with reduced survival in an adoptive transfer colitis model.47 Perhaps more strikingly, onset of disease was markedly accelerated in CA-MLCK transgenic mice. These data show that intestinal epithelial tight junction barrier loss can trigger mucosal immune activation, without causing disease, but can also enhance the rate of disease progression in a susceptible individual.
The data showing that a tight junction barrier defect enhances disease progression in a susceptible host may relate to human studies of post-infectious IBD and IBS,49, 50 where the barrier loss induced by infection may be the trigger that drives pathogenesis. Support for this comes from a recent study of GVHD. When CA-MLCK transgenic mice received cells from a minor antigen mismatched donor, they developed mild GVHD, while mice lacking the
CA-MLCK transgene remained healthy. Conversely, mice lacking the epithelial MLCK isoform (long MLCK) were protected from chronic GVHD, suggesting that targeted MLCK inhibition may be beneficial in GVHD.
Epithelial MLCK knockout mice have also been studied in the context of IBD.51 In this case, mice were significantly protected from adoptive transfer colitis and disease-associated barrier loss. However, epithelial MLCK knockout mice ultimately developed disease and barrier loss. Disease progression was associated with epithelial cell death and tight junction- independent barrier loss. This contrasts sharply with the durable protection from GVHD provided by epithelial MLCK knockout. The difference likely reflects an important distinction between advanced IBD and advanced GVHD. The former is characterized by epithelial damage and ulcers, while the latter typically has only rare apoptotic crypt epithelial cells. In addition to reinforcing the idea that barrier loss can be due to many processes other than tight junction dysregulation, these data suggest that MLCK inhibition may be helpful in mild IBD, or possibly in maintenance of remission, but is unlikely to be of benefit in advanced IBD.
Translation and Roadblocks
Implications for clinical diagnosis
The data above show that progress is being made in understanding barrier loss in disease, including and the means by which such barrier loss contributes to disease. However, they
also make it clear that intestinal barrier loss can occur by several mechanisms, only some of which reflect tight junction regulation, and that tight junction barrier loss can be divided into distinct pathways that are differentially modulated by disease effectors. Finally, increased intestinal permeability can be beneficial in some contexts, e.g. in promoting nutrient and water absorption or activating protective immunoregulatory processes. Thus, even if clinical assays that could measure intestinal barrier function more precisely than those available today were employed, it is not clear what could or should be done for a patient with increased intestinal permeability or even if the magnitude of permeability changes should be a factor in clinical decision-making. One case where an argument for therapeutic intervention might be made is that of a CD patient during clinical remission. Unfortunately, no barrier restorative therapies are available. In celiac disease and type I diabetes, data from mouse models suggest that restoring barrier function could also be beneficial, but there is no support from human trials. Finally, both human and mouse studies have made it clear that intestinal barrier loss alone, whether from tight junction dysregulation or epithelial damage, is insufficient to cause disease in an otherwise healthy individual.
Therapeutic approaches
For now, the best therapy for barrier loss should target the disease itself. For example, anti- TNF agents have been shown to restore barrier function in CD patients. For those complaining of leaky gut syndrome, the increased intestinal permeability present is as likely to reflect the underlying disorder as to be a cause of the pathogenic process. Thus, the leaky gut cures being sold at a variety of internet sites and alternative medicine stores should be considered with caution. None have been tested in randomized clinical trials, and they may do more harm than good.
Conclusions
Advances have been made in understanding the cellular mechanisms of intestinal barrier loss in disease. Unfortunately, the only agent purported to restore the barrier failed to do so in clinical trials. This may reflect the limited understanding of the mechanisms by which zonulin regulates barrier function. More detailed data are available for inflammatory mediators, such as TNF and IL-13, as well as some infectious agents. However, this information has not yet led to therapeutic agents suitable for clinical trials. MLCK could be a promising therapeutic target, but the inhibitors presently available target the MLCK enzymatic activity. Because the catalytic domain of MLCK is identical in epithelial and smooth muscle MLCK, toxicities of such an approach are likely to be unacceptable. Alternative means of specifically targeting intestinal epithelial MLCK must be sought if progress is to be made in this area. Similarly, no inhibitors of claudin-2 pore function are presently undergoing clinical trials. Finally, while a great deal has been learned about mechanisms of cell death, agents that prevent this in order to maintain barrier function have not been studied in humans. Thus, while much has been accomplished, further insight into both mechanisms of disease and development of novel therapeutic agents is needed before direct therapy of intestinal barrier function can be considered seriously.
References
1. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009; 9:799–809. [PubMed: 19855405]
2. Johansson ME, Phillipson M, Petersson J, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008; 105:15064–9. [PubMed: 18806221]
3. Levitt MD, Furne JK, Strocchi A, et al. Physiological measurements of luminal stirring in the dog and human small bowel. J Clin Invest. 1990; 86:1540–7. [PubMed: 2243130]
4. Van der Sluis M, De Koning BA, De Bruijn AC, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006; 131:117–29. [PubMed: 16831596]
5. Kottra G, Fromter E. Functional properties of the paracellular pathway in some leaky epithelia. J Exp Biol. 1983; 106:217–29. [PubMed: 6361205]
6. Shen L, Weber CR, Raleigh DR, et al. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011; 73:283–309. [PubMed: 20936941]
7. Francis SA, Kelly JM, McCormack J, et al. Rapid reduction of MDCK cell cholesterol by methyl- beta-cyclodextrin alters steady state transepithelial electrical resistance. Eur J Cell Biol. 1999; 78:473–84. [PubMed: 10472800]
8. Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010; 5:119–44. [PubMed: 20078218]
9. Schulzke JD, Ploeger S, Amasheh M, et al. Epithelial tight junctions in intestinal inflammation. Ann N Y Acad Sci. 2009; 1165:294–300. [PubMed: 19538319]
10. Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009; 1:a002584. [PubMed: 20066090]
11. Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol.
2006; 68:403–29. [PubMed: 16460278]
12. Tang VW, Goodenough DA. Paracellular ion channel at the tight junction. Biophys J. 2003; 84:1660–73. [PubMed: 12609869]
13. Madara JL. Increases in guinea pig small intestinal transepithelial resistance induced by osmotic loads are accompanied by rapid alterations in absorptive-cell tight-junction structure. J Cell Biol. 1983; 97:125–36. [PubMed: 6863387]
14. Madara JL, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol. 1986; 102:2125–36. [PubMed: 3711143]
15. Fihn BM, Sjoqvist A, Jodal M. Permeability of the rat small intestinal epithelium along the villus- crypt axis: effects of glucose transport. Gastroenterology. 2000; 119:1029–36. [PubMed: 11040189]
16. Clayburgh DR, Barrett TA, Tang Y, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005; 115:2702–15. [PubMed: 16184195]
17. Weber CR, Raleigh DR, Su L, et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010; 285:12037–46. [PubMed: 20177070]
18. Jodal M, Fihn B-M, Sjøqvist A. Effect of glucose on passive transport of extracellular probes across the rat small intestinal epithelium in vivo. Gastroenterology. 1994; 107:A241.
19. Watson CJ, Hoare CJ, Garrod DR, et al. Interferon-gamma selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J Cell Sci. 2005; 118:5221–30. [PubMed: 16249235]
20. Watson CJ, Rowland M, Warhurst G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am J Physiol Cell Physiol. 2001; 281:C388–97. [PubMed: 11443038]
21. Van Itallie CM, Holmes J, Bridges A, et al. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008; 121:298–305. [PubMed: 18198187]
22. Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005; 129:550–64. [PubMed: 16083712]
23. Amasheh S, Meiri N, Gitter AH, et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002; 115:4969–76. [PubMed: 12432083]
24. Rosenthal R, Milatz S, Krug SM, et al. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010; 123:1913–21. [PubMed: 20460438]
25. Zolotarevsky Y, Hecht G, Koutsouris A, et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002; 123:163–172. [PubMed: 12105845]
26. Blair SA, Kane SV, Clayburgh DR, et al. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006; 86:191–201. [PubMed: 16402035]
27. Krug SM, Fromm M, Gunzel D. Two-path impedance spectroscopy for measuring paracellular and transcellular epithelial resistance. Biophys J. 2009; 97:2202–11. [PubMed: 19843452]
28. Meddings JB, Gibbons I. Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology. 1998; 114:83–92. [PubMed: 9428222]
29. Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012; 303:G775–85. [PubMed: 22837345]
30. Rao AS, Camilleri M, Eckert DJ, et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol Gastrointest Liver Physiol. 2011; 301:G919–28. [PubMed: 21836056]
31. Turner JR, Cohen DE, Mrsny RJ, et al. Noninvasive in vivo analysis of human small intestinal paracellular absorption: Regulation by Na+-glucose cotransport. Dig Dis Sci. 2000; 45:2122–2126. [PubMed: 11215725]
32. Turner JR, Rill BK, Carlson SL, et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997; 273:C1378–85. [PubMed: 9357784]
33. Meddings JB, Westergaard H. Intestinal glucose transport using perfused rat jejunum in vivo: model analysis and derivation of corrected kinetic constants. Clin Sci (Lond). 1989; 76:403–13. [PubMed: 2714051]
34. Atisook K, Carlson S, Madara JL. Effects of phlorizin and sodium on glucose-elicited alterations of cell junctions in intestinal epithelia. Am J Physiol. 1990; 258:C77–C85. [PubMed: 2105653]
35. Ramakrishna BS, Venkataraman S, Srinivasan P, et al. Amylase-resistant starch plus oral rehydration solution for cholera. N Engl J Med. 2000; 342:308–13. [PubMed: 10655529]
36. Hollander D, Vadheim CM, Brettholz E, et al. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986; 105:883–5. [PubMed: 3777713]
37. May GR, Sutherland LM, Meddings JB. Lactulose/mannitol permeability is increased in relatives of patients with Crohn’s disease. Gastroenterology. 1992; 102:A934.
38. Buhner S, Buning C, Genschel J, et al. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut. 2006; 55:342–7. [PubMed: 16000642]
39. Wyatt J, Vogelsang H, Hubl W, et al. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993; 341:1437–9. [PubMed: 8099141]
40. D’Inca R, Di Leo V, Corrao G, et al. Intestinal permeability test as a predictor of clinical course in Crohn’s disease. Am J Gastroenterol. 1999; 94:2956–60. [PubMed: 10520851]
41. Bitton A, Dobkin P, Edwardes MD, et al. Predicting relapse in Crohn’s Disease: A biopsychosocial model. Gut. 2008
42. Meddings JB, Swain MG. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology. 2000; 119:1019–28. [PubMed: 11040188]
43. Boirivant M, Amendola A, Butera A, et al. A transient breach in the epithelial barrier leads to regulatory T-cell generation and resistance to experimental colitis. Gastroenterology. 2008; 135:1612–1623. [PubMed: 18765239]
44. Arrieta MC, Madsen K, Doyle J, et al. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009; 58:41–8. [PubMed: 18829978]
45. Watts T, Berti I, Sapone A, et al. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci U S A. 2005; 102:2916–21. [PubMed: 15710870]
46. Kelly CP, Green PHR, Murray JA, et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Alimentary Pharmacology
& Therapeutics. 2013; 37:252–262. [PubMed: 23163616]
47. Su L, Shen L, Clayburgh DR, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009; 136:551–63. [PubMed: 19027740]
48. May GR, Sutherland LR, Meddings JB. Is small intestinal permeability really increased in relatives of patients with Crohn’s disease? Gastroenterology. 1993; 104:1627–32. [PubMed: 8500719]
49. Gradel KO, Nielsen HL, Schonheyder HC, et al. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology. 2009; 137:495–501. [PubMed: 19361507]
50. Villani AC, Lemire M, Thabane M, et al. Genetic risk factors for post-infectious irritable bowel syndrome following a waterborne outbreak of gastroenteritis. Gastroenterology. 2010; 138:1502– 13. [PubMed: 20044998]
51. Su L, Nalle SC, Shen L, et al. TNFR2 Activates MLCK-Dependent Tight Junction Dysregulation to Cause Apoptosis-Mediated Barrier Loss and Experimental Colitis. Gastroenterology. 2013
52. Madsen KL, Malfair D, Gray D, et al. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999; 5:262– 70. [PubMed: 10579119]
53. Borody TJ, Warren EF, Leis S, et al. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003; 37:42–7. [PubMed: 12811208]
54. Summers RW, Elliott DE, Qadir K, et al. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003; 98:2034–41. [PubMed: 14499784]
55. Suenaert P, Bulteel V, Lemmens L, et al. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am J Gastroenterol. 2002; 97:2000–4. [PubMed: 12190167]
56. Scheinin T, Butler DM, Salway F, et al. Validation of the interleukin-10 knockout mouse model of colitis: antitumour necrosis factor-antibodies suppress the progression of colitis. Clin Exp Immunol. 2003; 133:38–43. [PubMed: 12823276]
57. Johansson JE, Ekman T. Gut toxicity during hemopoietic stem cell transplantation may predict acute graft-versus-host disease severity in patients. Dig Dis Sci. 2007; 52:2340–5. [PubMed: 17415646]
58. Brown GR, Lindberg G, Meddings J, et al. Tumor necrosis factor inhibitor ameliorates murine intestinal graft-versus-host disease. Gastroenterology. 1999; 116:593–601. [PubMed: 10029618]
59. Heidt PJ, Vossen JM. Experimental and clinical gnotobiotics: influence of the microflora on graft- versus-host disease after allogeneic bone marrow transplantation. J Med. 1992; 23:161–73. [PubMed: 1479298]
60. Beelen DW, Elmaagacli A, Muller KD, et al. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft- versus-host disease after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial. Blood. 1999; 93:3267–75. [PubMed: 10233878]
61. Bosi E, Molteni L, Radaelli MG, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006; 49:2824–7. [PubMed: 17028899]
62. Meddings JB, Jarand J, Urbanski SJ, et al. Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am J Physiol. 1999; 276:G951–7. [PubMed: 10198339]
63. Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008; 455:1109–13. [PubMed: 18806780]
64. Pozzilli P, Signore A, Williams AJ, et al. NOD mouse colonies around the world--recent facts and figures. Immunol Today. 1993; 14:193–6. [PubMed: 8517916]
65. Sharpstone D, Neild P, Crane R, et al. Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoea. Gut. 1999; 45:70–6. [PubMed: 10369707]
66. Keating J, Bjarnason I, Somasundaram S, et al. Intestinal absorptive capacity, intestinal permeability and jejunal histology in HIV and their relation to diarrhoea. Gut. 1995; 37:623–9. [PubMed: 8549936]
67. Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006; 12:1365–71. [PubMed: 17115046]
68. Epple HJ, Schneider T, Troeger H, et al. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut. 2009; 58:220–7. [PubMed: 18936106]
69. Doig CJ, Sutherland LR, Sandham JD, et al. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998; 158:444–51. [PubMed: 9700119]
70. Fink MP, Antonsson JB, Wang HL, et al. Increased intestinal permeability in endotoxic pigs.
Mesenteric hypoperfusion as an etiologic factor. Arch Surg. 1991; 126:211–8. [PubMed: 1899558]
71. Deitch EA, Morrison J, Berg R, et al. Effect of hemorrhagic shock on bacterial translocation, intestinal morphology, and intestinal permeability in conventional and antibiotic-decontaminated rats. Crit Care Med. 1990; 18:529–36. [PubMed: 2328600]
72. Dunlop SP, Hebden J, Campbell E, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006; 101:1288–94. [PubMed: 16771951]
73. Marshall JK, Thabane M, Garg AX, et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004; 20:1317–22. [PubMed: 15606393]
74. Smecuol E, Bai JC, Vazquez H, et al. Gastrointestinal permeability in celiac disease.
Gastroenterology. 1997; 112:1129–36. [PubMed: 9097995]
75. van Elburg RM, Uil JJ, Mulder CJ, et al. Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut. 1993; 34:354–7. [PubMed: 8472983]
76. Natividad JM, Huang X, Slack E, et al. Host responses to intestinal microbial antigens in gluten- sensitive mice. PLoS One. 2009; 4:e6472. [PubMed: 19649259]
77. Verdu EF, Huang X, Natividad J, et al. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2008; 294:G217–25. [PubMed: 18006603]
78. de Magistris L, Familiari V, Pascotto A, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010; 51:418–24. [PubMed: 20683204]
79. Pike MG, Heddle RJ, Boulton P, et al. Increased intestinal permeability in atopic eczema. J Invest Dermatol. 1986; 86:101–4. [PubMed: 3745938]
80. Jackson PG, Lessof MH, Baker RW, et al. Intestinal permeability in patients with eczema and food allergy. Lancet. 1981; 1:1285–6. [PubMed: 6112605]
81. Ammori BJ, Leeder PC, King RF, et al. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999; 3:252–62. [PubMed: 10481118]
82. Foitzik T, Fernandez-del Castillo C, Ferraro MJ, et al. Pathogenesis and prevention of early pancreatic infection in experimental acute necrotizing pancreatitis. Ann Surg. 1995; 222:179–85. [PubMed: 7639584]
83. Ryan CM, Schmidt J, Lewandrowski K, et al. Gut macromolecular permeability in pancreatitis correlates with severity of disease in rats. Gastroenterology. 1993; 104:890–5. [PubMed: 8440440]
84. Foitzik T, Stufler M, Hotz HG, et al. Glutamine stabilizes intestinal permeability and reduces pancreatic infection in acute experimental pancreatitis. J Gastrointest Surg. 1997; 1:40–6. discussion 46–7. [PubMed: 9834329]
85. Salat-Foix D, Tran K, Ranawaya R, et al. Increased intestinal permeability and Parkinson disease patients: chicken or egg? Can J Neurol Sci. 2012; 39:185–8. [PubMed: 22343151]
86. Davies KN, King D, Billington D, et al. Intestinal permeability and orocaecal transit time in elderly patients with Parkinson’s disease. Postgrad Med J. 1996; 72:164–7. [PubMed: 8731708]
87. Goebel A, Buhner S, Schedel R, et al. Altered intestinal permeability in patients with primary fibromyalgia and in patients with complex regional pain syndrome. Rheumatology (Oxford). 2008; 47:1223–7. [PubMed: 18540025]
88. Pimentel M, Wallace D, Hallegua D, et al. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann Rheum Dis. 2004; 63:450–2. [PubMed: 15020342]
89. Hijazi Z, Molla AM, Al-Habashi H, et al. Intestinal permeability is increased in bronchial asthma.
Arch Dis Child. 2004; 89:227–9. [PubMed: 14977697]
90. Umetsu DT, McIntire JJ, Akbari O, et al. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002; 3:715–20. [PubMed: 12145657]
91. Yacyshyn B, Meddings J, Sadowski D, et al. Multiple sclerosis patients have peripheral blood CD45RO+ B cells and increased intestinal permeability. Dig Dis Sci. 1996; 41:2493–8. [PubMed: 9011463]
92. Lee YK, Menezes JS, Umesaki Y, et al. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011; 108 (Suppl 1):4615–22. [PubMed: 20660719]
93. Martinez-Gonzalez O, Cantero-Hinojosa J, Paule-Sastre P, et al. Intestinal permeability in patients with ankylosing spondylitis and their healthy relatives. Br J Rheumatol. 1994; 33:644–7. [PubMed: 8019793]
94. Morris AJ, Howden CW, Robertson C, et al. Increased intestinal permeability in ankylosing spondylitis--primary lesion or drug effect? Gut. 1991; 32:1470–2. [PubMed: 1773950]
95. Taurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994; 180:2359–64. [PubMed: 7964509]
96. Wu HJ, Ivanov, Darce J, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010; 32:815–27. [PubMed: 20620945]
97. Wigg AJ, Roberts-Thomson IC, Dymock RB, et al. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001; 48:206–11. [PubMed: 11156641]
98. Farhadi A, Gundlapalli S, Shaikh M, et al. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 2008; 28:1026–33. [PubMed: 18397235]
99. Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology. 2000; 119:1340–7. [PubMed: 11054393]
100. Bergheim I, Weber S, Vos M, et al. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008; 48:983–92. [PubMed: 18395289]
101. Keshavarzian A, Holmes EW, Patel M, et al. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999; 94:200–7. [PubMed: 9934756]
102. Cesaro C, Tiso A, Del Prete A, et al. Gut microbiota and probiotics in chronic liver diseases. Dig Liver Dis. 2011; 43:431–8. [PubMed: 21163715]
Espero que sea algo esclarecedor, o por lo menos que sirva para que si os diagnostican de este sindrome, que las pruebas a realizar para su diagnóstico, sean lo suficientemente fiables
Saludos
